Organic compounds are the building blocks of life, making up the essential molecules that form living organisms. They are primarily composed of carbon (C) and hydrogen (H) atoms, often with the addition of other elements like oxygen (O), nitrogen (N), sulfur (S), and more. The unique feature of organic compounds is the presence of carbon-hydrogen (C-H) bonds, giving them distinct properties and versatility.
Carbon, with its ability to form strong covalent bonds with other atoms, serves as the backbone of organic molecules. Organic compounds are incredibly diverse and can range from simple, small molecules like methane (CH4) to complex, large biomolecules such as DNA and proteins. These compounds are found in all living organisms and play critical roles in various biological processes.
Examples of Organic Compounds
Organic compounds are all around us, in both natural and synthetic forms. Here are some common examples:
- Hydrocarbons: These are organic compounds made up of only carbon and hydrogen atoms. Examples include methane (CH4), ethane (C2H6), and benzene (C6H6).
- Alcohols: These compounds contain the hydroxyl (-OH) functional group. Common examples include ethanol (C2H5OH) and methanol (CH3OH).
- Aldehydes: Aldehydes are characterized by the carbonyl group (-CHO). Formaldehyde (CH2O) and acetaldehyde (CH3CHO) are examples.
- Ketones: Ketones have a carbonyl group within the carbon chain. Acetone (CH3COCH3) is a widely known ketone.
- Carboxylic Acids: These compounds feature the carboxyl group (-COOH). Examples include acetic acid (CH3COOH) and citric acid.
- Amines: Amines contain the amino group (-NH2). An example is ammonia (NH3).
- Esters: Esters are formed by the combination of a carboxylic acid and an alcohol. One well-known ester is ethyl acetate (CH3COOC2H5).
- Ethers: Ethers have the oxygen atom linking two carbon groups. Dimethyl ether (CH3OCH3) is an example.
Common Functional Groups
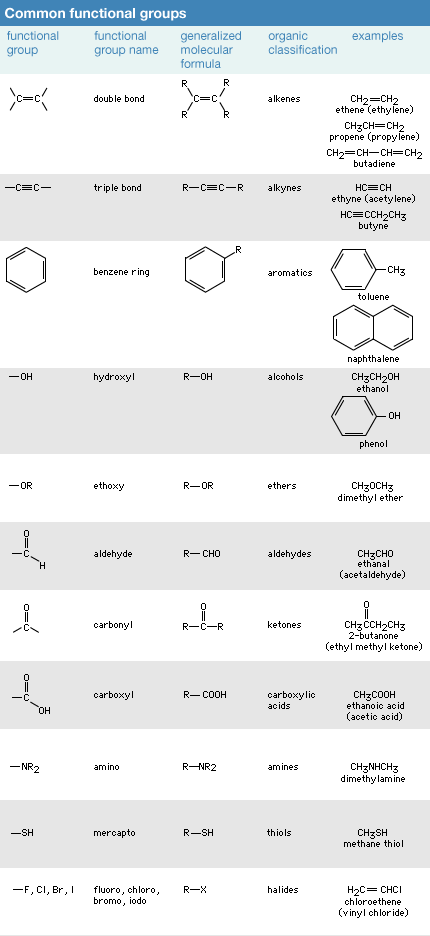
Functional groups are specific arrangements of atoms within organic compounds that impart distinct chemical properties to the molecule. Some common functional groups include:
- Hydroxyl (-OH): Found in alcohols, it imparts properties like solubility in water and the ability to form hydrogen bonds.
- Carbonyl (-CO): Present in aldehydes and ketones, it influences reactivity and polarity.
- Carboxyl (-COOH): Typical of carboxylic acids, it contributes acidity to the molecule.
- Amino (-NH2): Characteristic of amines, it imparts basicity and the ability to accept protons.
- Ester (-COO-): Found in esters, it is associated with pleasant odors and flavors in many natural compounds.
- Ether (-O-): Ethers are known for their low reactivity and use as solvents.
- Alkyl (-R): Alkyl groups consist of carbon and hydrogen atoms, serving as side chains in various organic molecules.
Acyclic or Open Chain Compounds
Acyclic or open chain compounds are organic molecules that do not form closed rings or cycles. They are often linear or branched structures. Acyclic compounds can be further categorized into saturated and unsaturated compounds:
- Saturated Compounds: These contain only single C-C bonds. Examples include alkanes like methane (CH4) and ethane (C2H6).
- Unsaturated Compounds: These contain one or more multiple bonds, such as double (C=C) or triple (C≡C) bonds. Examples include alkenes (e.g., ethene, C2H4) and alkynes (e.g., ethyne, C2H2).
Alicyclic or Closed Chain or Ring Compounds
Alicyclic compounds, also known as closed chain or ring compounds, are organic molecules characterized by one or more carbon rings in their structure. These rings can vary in size and complexity, and they often exhibit unique properties. Some examples include cycloalkanes, which are saturated ring compounds like cyclohexane (C6H12), and cycloalkenes, which are unsaturated ring compounds like cyclohexene (C6H10).
Aromatic Compounds
Aromatic compounds are a class of organic compounds known for their distinctive, stable ring structure and a set of shared properties. They are characterized by a phenomenon called aromaticity, which results from a resonance stabilization of electrons within the ring. Aromatic compounds can be further divided into two categories:
Benzenoid aromatic compounds possess a benzene ring, a six-membered carbon ring with alternating double bonds. Benzene (C6H6) itself is the classic example of a benzenoid aromatic compound.
Non-benzenoid aromatic compounds are aromatic compounds that do not have a benzene ring but exhibit aromaticity due to resonance structures. Pyridine (C5H5N) is a common example.
Heterocyclic Aromatic Compounds
Heterocyclic aromatic compounds are a subset of aromatic compounds where one or more carbon atoms in the ring are replaced by heteroatoms like nitrogen, oxygen, or sulfur. These compounds are widely distributed in nature and are essential components of many biomolecules and pharmaceuticals. Examples include pyrrole, furan, and thiophene, which contain nitrogen, oxygen, and sulfur in their respective five-membered rings.
Classification of Organic Compounds
Organic compounds can be classified based on various criteria, including their structure, functional groups, and properties.
Some common classification schemes include:
- Functional Group-Based Classification: Organic compounds can be categorized into groups like hydrocarbons, alcohols, ketones, and carboxylic acids based on the presence of specific functional groups. This classification helps chemists predict the chemical behavior of compounds and their reactivity.
- Structural Classification: Organic compounds can be classified based on their structural features, such as acyclic or cyclic, branched or unbranched, and the presence of rings or chains. This classification aids in understanding the geometry and connectivity of atoms within a molecule.
- Degree of Saturation: Organic compounds can be classified as saturated or unsaturated based on the number of carbon-carbon bonds. Saturated compounds have only single bonds (e.g., alkanes), while unsaturated compounds have double or triple bonds (e.g., alkenes and alkynes).
- Carbon Skeleton: Organic compounds can be categorized by the length and complexity of their carbon backbone. This includes straight-chain (linear), branched-chain, and cyclic compounds, each with its own set of chemical properties.
- Functional Use: Compounds can be classified by their practical applications, such as pharmaceuticals, fuels, solvents, plastics, and natural products. This classification is important for industrial and commercial purposes.
- Biological Classification: Organic compounds found in living organisms can be classified into categories like carbohydrates, lipids, proteins, and nucleic acids. This classification is crucial for understanding the biochemical processes of life.
- Aromatic vs. Non-Aromatic: Aromatic compounds, as mentioned earlier, are a distinct class characterized by their unique ring structure and resonance stability. Non-aromatic compounds lack these characteristics.
- Heteroatoms: Some compounds are classified based on the presence of heteroatoms (atoms other than carbon and hydrogen) like nitrogen, oxygen, or sulfur. This classification is common in the context of heterocyclic compounds.
In conclusion, organic compounds are incredibly diverse and form the foundation of life as we know it. They exhibit a wide range of structures, properties, and functions, making them central to fields such as chemistry, biology, and industry. Understanding the classification and characteristics of organic compounds is essential for scientists and researchers in various domains, as it enables the manipulation and utilization of these compounds for a multitude of applications, from the development of new drugs to the creation of sustainable materials.

Here are some frequently asked questions (FAQs) related to organic compounds:
What are organic compounds, and why are they important?
Organic compounds are molecules primarily composed of carbon and hydrogen atoms, often with other elements like oxygen, nitrogen, or sulfur. They are crucial because they form the foundation of life, making up the essential molecules in living organisms. Organic compounds play vital roles in various biological processes and have diverse applications in industry and chemistry.
What are some common examples of organic compounds?
Common examples of organic compounds include hydrocarbons like methane and ethane, alcohols like ethanol, carboxylic acids like acetic acid, and many more. Organic compounds are present in everyday items, from fuels and plastics to pharmaceuticals and natural products.
What are functional groups in organic chemistry?
Functional groups are specific arrangements of atoms within organic compounds that give them distinct chemical properties. They determine how a molecule will react and interact with other substances. Common functional groups include hydroxyl (-OH), carbonyl (-CO), carboxyl (-COOH), amino (-NH2), and ester (-COO-).
How are organic compounds classified?
Organic compounds can be classified based on various criteria, including their structure, functional groups, degree of saturation (saturated or unsaturated), carbon skeleton (linear, branched, or cyclic), and practical applications (pharmaceuticals, fuels, plastics). Additionally, they can be categorized as aromatic or non-aromatic and based on the presence of heteroatoms.
What is the difference between acyclic and alicyclic compounds?
Acyclic or open chain compounds do not have closed rings in their structure and can be linear or branched. Alicyclic compounds, on the other hand, contain one or more closed carbon rings, which can be saturated (cycloalkanes) or unsaturated (cycloalkenes).