Organic chemistry can seem daunting, but understanding functional groups is a key step in making it more approachable. In this article, we'll delve into the world of organic chemistry functional groups, breaking down complex molecules into manageable components. By the end, you'll have a solid grasp of what functional groups are, how they affect chemical reactions, and their significance in the world of organic chemistry.
Unlocking the World of Functional Groups
Organic chemistry is the study of carbon-containing compounds, and functional groups are the building blocks of these compounds. They are specific groups of atoms attached to a carbon backbone that give organic molecules their unique properties. Functional groups are like the LEGO bricks of organic chemistry, allowing chemists to create a wide variety of molecules with different functions and characteristics.
The Alkane Family: Simplicity in Hydrocarbons
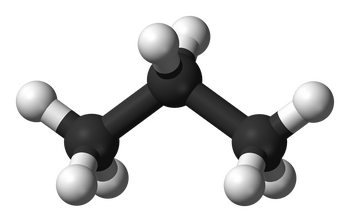
Alkanes are often the starting point when discussing organic chemistry and functional groups because they represent the simplest of organic compounds. They are composed solely of carbon and hydrogen atoms connected by single covalent bonds, which is a characteristic that sets them apart from other functional groups.
Structure of Alkanes: Hydrogen and Carbon Harmony
Alkanes have a straightforward structure. Carbon atoms in alkanes are tetrahedral, meaning they form a three-dimensional shape with bond angles of approximately 109.5 degrees. Each carbon atom is bonded to four other atoms, either carbon or hydrogen, via single covalent bonds. This results in a straight or branched carbon chain.
Saturated Hydrocarbons: The Absence of Functional Groups
Alkanes are often referred to as saturated hydrocarbons because they are "saturated" with hydrogen atoms, meaning there are no other functional groups or elements present. This saturation gives alkanes their relative inertness; they don't readily participate in most chemical reactions, making them stable and unreactive compared to other functional groups.
Common Alkanes: Everyday Hydrocarbons
Alkanes are not just abstract compounds; they're present in our daily lives. Some common alkanes include methane (CH4), ethane (C2H6), propane (C3H8), and butane (C4H10). Methane, in particular, is the primary component of natural gas, and butane can be found in lighter fluid and aerosol propellants. Their widespread use showcases the simplicity and stability of alkanes.
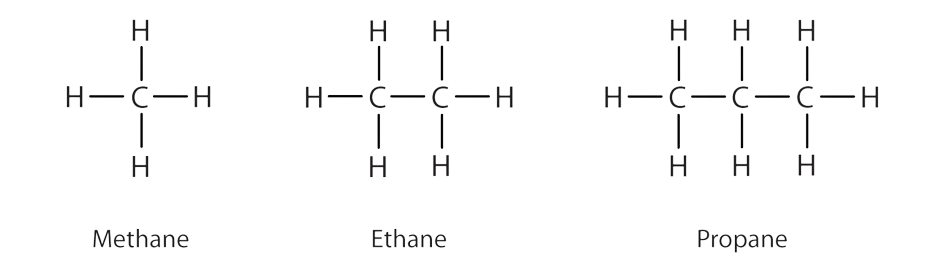
Uses of Alkanes
Despite their relatively unreactive nature, alkanes find use in various applications. For example:
- Fuel: Alkanes, especially longer chain ones like octane, are components of gasoline, which powers most internal combustion engines.
- Lubricants: Some alkanes are used as lubricants due to their non-reactive nature.
- Heating: Alkanes like natural gas and propane are used for heating and cooking in many households.
Understanding Alkanes in Organic Chemistry
While alkanes themselves may seem unexciting in terms of chemical reactivity, they serve as a crucial starting point in organic chemistry. When discussing more complex functional groups, it's essential to understand that they often build upon the backbone of alkane structures. The simplicity and stability of alkanes provide a solid foundation for studying how functional groups influence and modify the behavior of organic compounds.
Hydroxyl Group (-OH): The Alcohol's Signature
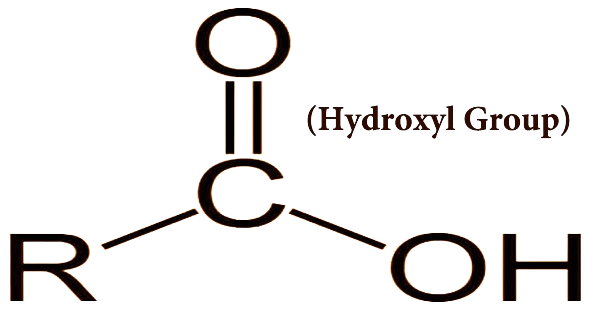
The hydroxyl group, represented as -OH, is one of the most recognizable and essential functional groups in organic chemistry. It's famously associated with alcohol, and its presence imparts unique properties to the compounds that contain it.
Structure of the Hydroxyl Group
The hydroxyl group consists of an oxygen atom (O) bonded to a hydrogen atom (H) and attached to a carbon atom in the organic molecule. This oxygen-hydrogen pair forms a polar covalent bond, with oxygen being more electronegative than hydrogen. As a result, oxygen carries a partial negative charge, and hydrogen carries a partial positive charge. This polarity makes the -OH bond in hydroxyl groups highly reactive and capable of forming hydrogen bonds.
Solubility and Hydrogen Bonding
One of the key characteristics of compounds containing hydroxyl groups is their solubility in water. The polarity of the -OH bond allows it to form hydrogen bonds with water molecules. These hydrogen bonds facilitate the dissolution of alcohols in water, making them miscible in a wide range of aqueous solutions.
Alcohols: The Most Familiar Hydroxyl-Containing Compounds
Alcohols are organic compounds characterized by the presence of one or more hydroxyl groups. They are ubiquitous in both everyday life and laboratory settings. Ethanol (C2H5OH), for example, is the active ingredient in alcoholic beverages. Methanol (CH3OH) and isopropanol (C3H7OH) are common industrial solvents and disinfectants.
Chemical Properties of Hydroxyl Groups
Hydroxyl groups confer unique chemical reactivity on compounds.
They can undergo various reactions, including:
- Oxidation: The -OH group can be oxidized to form a carbonyl group (C=O) in compounds like aldehydes and ketones.
- Ester Formation: Alcohols can react with carboxylic acids to form esters, which are essential components in many fragrances and flavor compounds.
- Dehydration: Under suitable conditions, two hydroxyl groups in neighboring molecules can lose water, forming an ether linkage.
Biological Significance of Hydroxyl Groups
In biology, hydroxyl groups are critical components of many biomolecules. They are found in carbohydrates (e.g., glucose), nucleic acids (e.g., DNA and RNA), and various secondary metabolites in plants. The presence of hydroxyl groups in these molecules influences their structure, solubility, and interactions with other biomolecules.
Carbonyl Group (C=O): A Versatile Functional Group
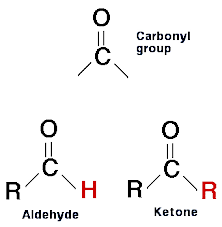
The carbonyl group, represented as C=O, is one of the most versatile and prevalent functional groups in organic chemistry. It is found in a wide range of compounds, including aldehydes, ketones, carboxylic acids, esters, amides, and more. The carbonyl group consists of a carbon atom (C) double-bonded to an oxygen atom (O).
Structure and Reactivity of the Carbonyl Group
The carbonyl group is characterized by a polarized double bond between carbon and oxygen. Oxygen is more electronegative than carbon, resulting in a partial negative charge on the oxygen atom and a partial positive charge on the carbon atom. This polarization makes the carbonyl carbon electrophilic, meaning it is eager to accept electrons in chemical reactions.
Aldehydes: The First Class of Carbonyl Compounds
In aldehydes, the carbonyl group is found at the end of a carbon chain. Formaldehyde (H2C=O) is the simplest aldehyde, followed by acetaldehyde (CH3C=O). Aldehydes are known for their distinctive, sometimes pungent odors, and they are essential in various chemical reactions, including the synthesis of alcohols.
Ketones: The Carbonyl Group Within Carbon Chains
Ketones have the carbonyl group within the carbon chain. Acetone (CH3COCH3) is one of the most well-known ketones and is commonly used as a solvent and in nail polish removers. Ketones play significant roles in organic synthesis, particularly in the formation of secondary alcohols.
Chemical Reactions of the Carbonyl Group
The carbonyl group is involved in a broad spectrum of chemical reactions:
- Nucleophilic Addition: The electron-rich part of a molecule, called a nucleophile, can attack the electrophilic carbon atom of the carbonyl group. This leads to the formation of alcohol or related compounds.
- Oxidation and Reduction: The carbonyl group can be oxidized to a carboxylic acid or reduced to an alcohol, making it a crucial player in redox reactions.
- Tautomerization: Carbonyl compounds can undergo tautomerization, where the position of the double bond shifts, converting a ketone into an enol, or an aldehyde into an enol.
Practical Applications
The versatility of carbonyl groups makes them invaluable in organic synthesis, pharmaceuticals, and the flavor and fragrance industry. They are vital in creating a wide array of compounds, from pharmaceutical drugs to fragrances in perfumes and flavors in foods.
Carboxyl Group (-COOH): The Acids and Beyond
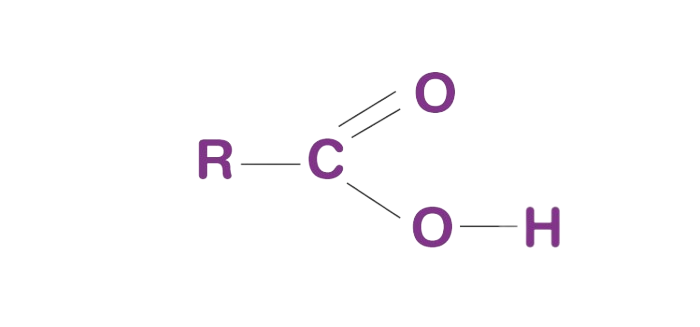
The carboxyl group, represented as -COOH, is a fundamental functional group in organic chemistry, and it's the defining feature of carboxylic acids. This group plays a crucial role in the world of organic compounds, particularly in the realm of acidity and chemical reactivity.
Structure of the Carboxyl Group
The carboxyl group consists of a carbonyl group (C=O) bonded to a hydroxyl group (-OH) at the same carbon atom. This carbon atom is also bonded to an oxygen atom in another direction. The oxygen in the hydroxyl group is polar, contributing to the overall polarity of the -COOH group.
Carboxylic Acids: The Producers of Hydrogen Ions
Carboxylic acids are the most common compounds that contain a carboxyl group. They are characterized by their ability to release hydrogen ions (H+) into solution when dissolved in water. This property makes them weak acids, capable of lowering the pH of a solution.
Acidity and pKa: Measuring Strength
The strength of carboxylic acids can vary, and it is often quantified using a value known as pKa. Lower pKa values indicate stronger acids. The presence of the -COOH group imparts acidity, with carboxylic acids like acetic acid (CH3COOH) and citric acid (found in citrus fruits) demonstrating this property.
Reactions of Carboxylic Acids
Carboxylic acids can participate in various chemical reactions:
- Esterification: They can react with alcohols to form esters, a process used in the production of fragrances, flavors, and plasticizers.
- Salt Formation: In a neutralization reaction with a base, carboxylic acids can form carboxylate salts, where the -OH group becomes -O-.
- Decarboxylation: Under specific conditions, carboxylic acids can lose the carboxyl group, leading to the formation of other compounds. This reaction plays a role in the biosynthesis of various natural products.
Amino Group (-NH2): The Building Blocks of Life
The amino group, represented as -NH2, is a crucial functional group in organic chemistry, playing a fundamental role in the molecular structures of life. It is the defining feature of amino acids, which serve as the building blocks of proteins, and it contributes to the diversity of biomolecules.
Structure of the Amino Group
The amino group consists of a nitrogen atom (N) bonded to two hydrogen atoms (H). This group is often attached to a carbon atom, forming an amino acid. In amino acids, the amino group combines with a carboxyl group (-COOH), creating a zwitterion, a molecule with both a positive and negative charge. This duality of charge allows amino acids to function as both acids and bases.
Amino Acids: Protein Building Blocks
Amino acids are organic compounds that serve as the fundamental building blocks of proteins. There are 20 standard amino acids used in protein synthesis. They differ from one another based on the unique side chain or "R-group" attached to the central carbon atom. These side chains determine the specific properties of each amino acid and influence the function of the protein they create.
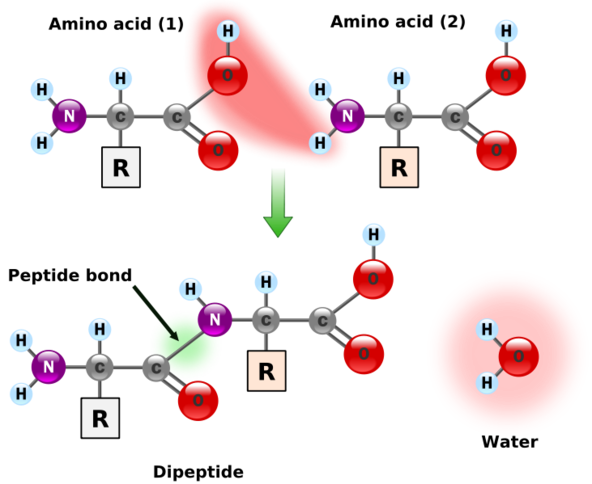
Peptide Bonds: Linking Amino Acids
Amino acids are linked together in proteins through peptide bonds, a type of covalent bond formed by a dehydration synthesis reaction. The amino group of one amino acid reacts with the carboxyl group of another, resulting in the loss of a water molecule and the formation of a strong peptide bond. This process continues to create the linear chains of amino acids that constitute proteins.
Proteins: Diverse Functions in Life
Proteins are essential macromolecules with diverse functions in living organisms. They serve as enzymes and catalysts that speed up chemical reactions. Proteins also provide structural support in tissues and cells, transmit signals, transport molecules, and act as antibodies in the immune system. The wide variety of functions that proteins perform is a testament to the diversity of amino acids and the structural versatility provided by the amino group.
Phosphate Group (-PO4): Energy Currency of Cells
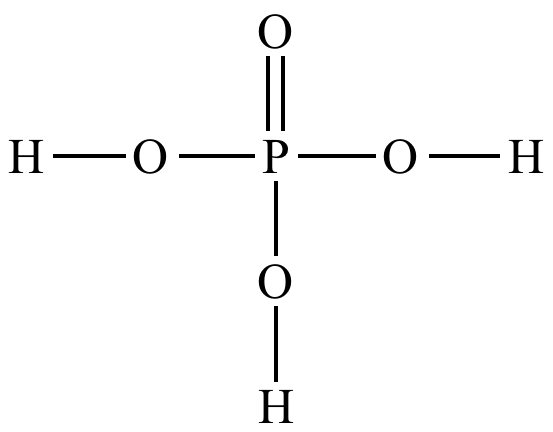
The phosphate group, represented as -PO4, is a fundamental functional group in organic chemistry. In the context of cellular biology, it plays a pivotal role as a primary component of adenosine triphosphate (ATP), which is often referred to as the energy currency of cells.
Structure of the Phosphate Group
The phosphate group consists of a central phosphorus atom (P) bonded to four oxygen atoms (O), with one oxygen atom bonded directly to the phosphorus atom and the other three oxygen atoms connected to the phosphorus through single or double bonds. These oxygen atoms can carry negative charges, which give the phosphate group its high reactivity and make it a versatile player in biochemical reactions.
Adenosine Triphosphate (ATP): Energy Storage and Transfer
Adenosine triphosphate, or ATP, is a molecule that stores and transfers energy within cells. It consists of an adenine base, a ribose sugar, and three phosphate groups, linked together in a chain. The key to ATP's role as an energy currency lies in the high-energy phosphate bonds that link the phosphate groups. When these bonds are broken in a hydrolysis reaction, energy is released and can be used to power cellular processes.
ATP: Energy Transfer and Cellular Work
In cells, ATP serves as a universal energy carrier. It powers various cellular processes, including:
- Muscle Contraction: ATP provides the energy required for muscle fibers to contract.
- Active Transport: Cells use ATP to move molecules against concentration gradients, a process known as active transport.
- Enzyme Catalysis: ATP is essential in enzyme-catalyzed reactions, where it acts as a source of phosphate groups that can be transferred to other molecules, facilitating chemical reactions.
- Nucleic Acid Synthesis: ATP is involved in DNA and RNA synthesis, providing the necessary energy for polymerization.
Phosphorylation: The Addition of Phosphate Groups
The transfer of phosphate groups from ATP to other molecules is a crucial process in cellular regulation and signaling. This process is called phosphorylation. By attaching phosphate groups to specific target molecules, cells can alter their activity and function.
Phosphate Group in Nucleic Acids
Phosphate groups are also essential components of nucleic acids like DNA and RNA. In these molecules, the phosphate group connects the sugar and nucleotide units, forming the backbone of the nucleic acid chains.
Conclusion: Functional Groups Unveiled
In organic chemistry, functional groups are the puzzle pieces that, when combined, create a magnificent picture of diverse molecules. Understanding these groups and their properties allows chemists to predict how compounds will react and design new molecules with specific functions. While this article only scratches the surface of functional groups, it provides a solid foundation for further exploration. So, the next time you encounter a complex organic molecule, remember that it's made up of these remarkable functional groups, each with its own unique role in the chemical orchestra of life.
By breaking down the complexity of organic molecules into their functional groups, we can demystify the intricate world of organic chemistry. These groups are not just abstract concepts; they are the keys to understanding the behavior of organic compounds, from the simplest alkanes to the most complex biomolecules. With this newfound knowledge, you can embark on a journey to explore the fascinating realm of organic chemistry with confidence and curiosity.

Here are some frequently asked questions (FAQs) related to organic chemistry functional groups:
What are functional groups in organic chemistry?
Functional groups are specific groups of atoms attached to a carbon backbone in organic molecules. They determine the chemical properties and reactivity of the compound.
Why are functional groups important in organic chemistry?
Functional groups dictate how organic compounds interact with other substances and participate in chemical reactions. They are essential for understanding and predicting the behavior of organic molecules.
How are functional groups classified?
Functional groups can be classified into different categories, such as hydrocarbons (e.g., alkane family), oxygen-containing groups (e.g., hydroxyl and carbonyl), nitrogen-containing groups (e.g., amino), and phosphorus-containing groups (e.g., phosphate).
What is the significance of hydroxyl groups (-OH) in organic molecules?
Hydroxyl groups make molecules like alcohols soluble in water and enable them to form hydrogen bonds. They are vital in various chemical and biological processes.
How do carbonyl groups (C=O) contribute to the reactivity of compounds?
Carbonyl groups are found in aldehydes and ketones and play a role in oxidation and reduction reactions. They are versatile and participate in numerous chemical transformations.