The Medical College Admission Test (MCAT) is a crucial exam for aspiring medical professionals, encompassing various scientific disciplines. One fundamental aspect of the MCAT is its reliance on the periodic table of elements. This iconic arrangement of chemical elements provides a comprehensive framework for understanding the properties, trends, and relationships among elements. In this blog, we will explore the pivotal role of the periodic table on the MCAT and how it assists students in tackling questions related to chemistry and other sciences.
Historical Perspective
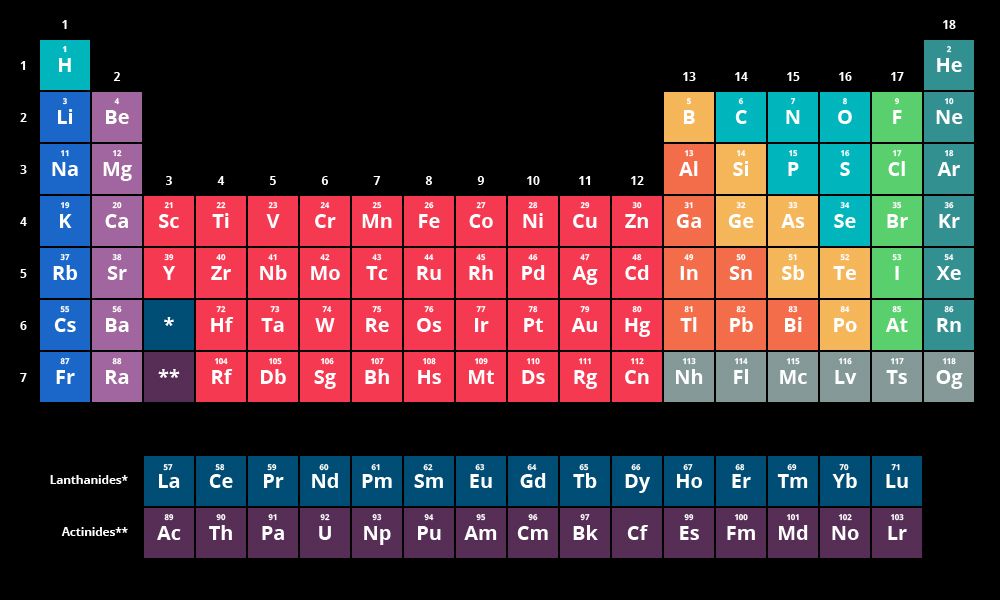
Before delving into the significance of the periodic table on the MCAT, it is essential to understand its historical context. Developed by Dmitri Mendeleev in 1869, the periodic table revolutionized the field of chemistry. Mendeleev's table organized elements based on their atomic mass, enabling him to predict the existence of elements yet to be discovered. Over time, the periodic table evolved as new elements were discovered and our understanding of atomic structure advanced. Today, the table organizes elements based on their atomic number, reflecting the number of protons in an atom's nucleus.
Fundamental Concepts
The periodic table serves as a foundational tool for MCAT test-takers to grasp several key concepts. It allows students to identify an element's atomic number, atomic symbol, atomic mass, and electron configuration. Furthermore, it provides insights into an element's group and period, which are critical for understanding trends in chemical and physical properties.
Periodic trends, such as atomic radius, ionization energy, electronegativity, and electron affinity, are crucial aspects tested on the MCAT. The periodic table's structure helps students recognize these trends and make predictions based on the element's position within the table. For instance, as one moves across a period from left to right, atomic radius decreases, while ionization energy and electronegativity increase.
Chemical Reactions and Bonding
Understanding chemical reactions and bonding is essential for success on the MCAT, and the periodic table provides the necessary foundation for these topics. The table allows students to identify the valence electrons of each element, which determines its reactivity and bonding behavior.
Valence electrons play a vital role in the formation of chemical bonds, including ionic, covalent, and metallic bonds. By referencing the periodic table, students can predict how many electrons an element needs to gain or lose to achieve a stable electron configuration. This knowledge is crucial for comprehending and predicting chemical reactions, particularly those involving elements from different groups.
The periodic table also facilitates understanding the concept of periodicity in chemical reactions. The reactivity of elements often follows predictable patterns based on their position within the table. For instance, alkali metals in Group 1 readily lose their valence electrons, while halogens in Group 17 easily gain electrons to achieve a stable configuration.
Biological Applications
The MCAT assesses students' knowledge of biology and its intersection with chemistry. Many biological processes rely on the interaction of elements and compounds. The periodic table aids in comprehending biochemistry and physiology-related questions by providing insights into the elements that constitute biological molecules.
Understanding the distribution of elements in the human body, such as carbon, oxygen, nitrogen, hydrogen, and phosphorus, is crucial for comprehending various biological processes. The periodic table highlights the abundance and significance of these elements, aiding students in understanding topics such as protein structure, DNA replication, and enzyme activity.
Moreover, the periodic table helps students understand the behavior of ions in biological systems. Many physiological processes involve ion channels and electrochemical gradients, which depend on the movement of charged particles. By referencing the periodic table, students can determine the charge and oxidation states of elements, which is essential for understanding the role of ions in biological systems.
Analytical Techniques
The MCAT also tests students' understanding of analytical techniques used in chemistry and biochemistry. The periodic table is instrumental in comprehending these techniques and their applications. For example, spectroscopy, a commonly used analytical method, relies on the identification of elements based on their unique patterns of atomic emission or absorption. By understanding the periodic table and the properties associated with specific elements, students can interpret spectroscopic data and identify unknown compounds.
Similarly, the periodic table assists in understanding other analytical techniques, such as chromatography and mass spectrometry. These techniques rely on the separation and identification of compounds based on their chemical properties and mass-to-charge ratios, respectively. The periodic table provides valuable information about the elements and isotopes involved, aiding students in interpreting the results obtained from these techniques.
Practical Tips for MCAT Success
To excel in the MCAT, it is crucial to develop a solid understanding of the periodic table and its applications. Here are some practical tips to help you navigate this important resource:
Conclusion
The periodic table plays a pivotal role in the MCAT, providing a framework for understanding the properties, trends, and relationships among elements. Its importance extends beyond chemistry, as it aids in comprehending topics in biology, biochemistry, analytical techniques, and more. By mastering the periodic table, students can confidently tackle MCAT questions related to chemical reactions, bonding, periodic trends, spectroscopy, and other scientific concepts. Understanding the layout, key trends, and practical applications of the periodic table is essential for success on the MCAT and lays a solid foundation for future medical professionals.

Here are the FAQs About MCAT
Why is the periodic table important on the MCAT?
The periodic table is essential on the MCAT because it serves as a foundational tool for understanding concepts related to chemistry, biology, and analytical techniques. It helps students identify elements, determine their properties, predict trends, comprehend chemical reactions, and analyze spectroscopic data. Mastering the periodic table is crucial for success on the MCAT.
How should I study and memorize the periodic table for the MCAT?
To study and memorize the periodic table, start by familiarizing yourself with its structure, including periods, groups, and blocks. Understand the information provided for each element, such as atomic symbol, atomic number, atomic mass, and electron configuration. Practice identifying elements based on their symbols, numbers, and configurations. Additionally, memorize key trends in atomic properties and make connections between elements and their biological applications.
What are some important periodic trends to understand for the MCAT?
Some important periodic trends to understand for the MCAT include atomic radius, ionization energy, electronegativity, and electron affinity. Recognizing how these properties change across periods and groups helps predict reactivity, bonding behavior, and chemical reactions. Understanding these trends is crucial for answering questions related to periodicity and chemical properties on the MCAT.
How does the periodic table relate to biology on the MCAT?
The periodic table relates to biology on the MCAT through its association with elements that are essential to biological processes. Elements such as carbon, oxygen, nitrogen, hydrogen, and phosphorus play vital roles in biological molecules, protein structure, DNA replication, enzyme activity, and ion channels. The periodic table helps students understand the distribution, abundance, and significance of these elements in the human body and their relevance to various biological systems.
How does the periodic table assist in analytical techniques on the MCAT?
The periodic table assists in analytical techniques on the MCAT by providing information about elements and isotopes used in these techniques. Spectroscopy relies on the identification of elements based on their unique emission or absorption patterns, while chromatography and mass spectrometry involve the separation and identification of compounds based on their chemical properties and mass-to-charge ratios. The periodic table helps students interpret spectroscopic data and understand the elements involved in these techniques.
What are some practical tips for success with the periodic table on the MCAT?
To succeed with the periodic table on the MCAT, familiarize yourself with its structure, layout, and information provided for each element. Memorize key trends in atomic properties and practice identifying elements quickly and accurately. Make connections between the periodic table and other scientific disciplines, such as biology and analytical techniques. Regular practice and understanding of the practical applications of the periodic table will enhance your performance on MCAT questions related to chemistry, biology, and analytical concepts.





